How Do Buffers Work Chemistry . how does a buffer work? how a buffer works. Buffers function through a process of chemical equilibrium. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. An example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. how do buffer solutions work? When you add an acid to a buffer, the conjugate base. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is able to resist ph change because the two components (conjugate acid and. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. Ions are atoms or molecules that have lost or gained one or more electrons.
from slidetodoc.com
how does a buffer work? To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. Buffers function through a process of chemical equilibrium. When you add an acid to a buffer, the conjugate base. A buffer is able to resist ph change because the two components (conjugate acid and. how a buffer works. Ions are atoms or molecules that have lost or gained one or more electrons. An example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. how do buffer solutions work?
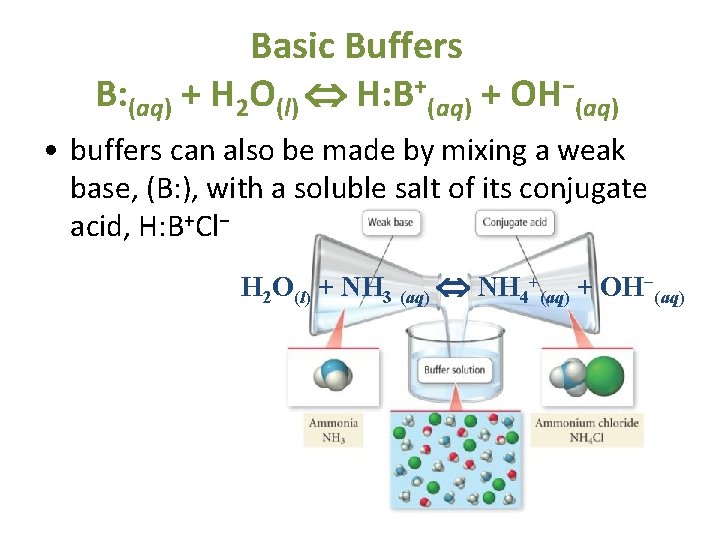
Introduction to Buffers These solutions contain relatively high
How Do Buffers Work Chemistry Buffers function through a process of chemical equilibrium. how do buffer solutions work? how a buffer works. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. Ions are atoms or molecules that have lost or gained one or more electrons. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Buffers function through a process of chemical equilibrium. An example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. how does a buffer work? To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. When you add an acid to a buffer, the conjugate base. A buffer is able to resist ph change because the two components (conjugate acid and.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium How Do Buffers Work Chemistry Buffers function through a process of chemical equilibrium. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. how do buffer solutions work? An example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. To illustrate the function of a buffer solution, consider a mixture of. How Do Buffers Work Chemistry.
From saylordotorg.github.io
Buffers How Do Buffers Work Chemistry Buffers function through a process of chemical equilibrium. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is able to resist ph change because the two components (conjugate acid and. When you add an acid to a buffer, the conjugate base. how. How Do Buffers Work Chemistry.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation How Do Buffers Work Chemistry Buffers function through a process of chemical equilibrium. Ions are atoms or molecules that have lost or gained one or more electrons. When you add an acid to a buffer, the conjugate base. An example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. A buffer is able to resist ph change because the two components. How Do Buffers Work Chemistry.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk How Do Buffers Work Chemistry how do buffer solutions work? Ions are atoms or molecules that have lost or gained one or more electrons. An example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. how a buffer works. When you add an acid to a buffer, the conjugate base. buffer, in chemistry, solution usually containing an acid. How Do Buffers Work Chemistry.
From www.youtube.com
How Chemical Buffers Work & Buffers in Natural Systems // HSC Chemistry How Do Buffers Work Chemistry A buffer is able to resist ph change because the two components (conjugate acid and. Buffers function through a process of chemical equilibrium. Ions are atoms or molecules that have lost or gained one or more electrons. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion. How Do Buffers Work Chemistry.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG How Do Buffers Work Chemistry how do buffer solutions work? Ions are atoms or molecules that have lost or gained one or more electrons. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. When you add an acid to a buffer, the conjugate base. Buffers function through a process. How Do Buffers Work Chemistry.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation How Do Buffers Work Chemistry An example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. A buffer is able to resist ph change because the two components (conjugate acid and. When you add an acid to a buffer, the conjugate base. Buffers function through a process of chemical equilibrium. To illustrate the function of a buffer solution, consider a mixture. How Do Buffers Work Chemistry.
From www.slideserve.com
PPT All About Buffers PowerPoint Presentation, free download ID1321295 How Do Buffers Work Chemistry how does a buffer work? buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. When you add an acid to a buffer, the conjugate base. A buffer is able to resist ph change because the two components (conjugate acid and. how a buffer. How Do Buffers Work Chemistry.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination for Biologists How Do Buffers Work Chemistry A buffer is able to resist ph change because the two components (conjugate acid and. When you add an acid to a buffer, the conjugate base. Ions are atoms or molecules that have lost or gained one or more electrons. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. . How Do Buffers Work Chemistry.
From sciencenotes.org
Buffer Definition and Examples in Chemistry How Do Buffers Work Chemistry buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. An example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. A buffer. How Do Buffers Work Chemistry.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its How Do Buffers Work Chemistry Ions are atoms or molecules that have lost or gained one or more electrons. how does a buffer work? how do buffer solutions work? To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. When you add an acid to a buffer, the conjugate base. To illustrate the function. How Do Buffers Work Chemistry.
From www.slideserve.com
PPT 18 Additional Aspects of AcidBase Equilibria PowerPoint How Do Buffers Work Chemistry how do buffer solutions work? Ions are atoms or molecules that have lost or gained one or more electrons. how a buffer works. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. When you add an acid to a buffer, the conjugate base. buffer, in chemistry, solution. How Do Buffers Work Chemistry.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 How Do Buffers Work Chemistry Ions are atoms or molecules that have lost or gained one or more electrons. Buffers function through a process of chemical equilibrium. how do buffer solutions work? how does a buffer work? how a buffer works. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant. How Do Buffers Work Chemistry.
From goldbio.com
What is a Biological Buffer and How to Choose the Best Buffer for Your How Do Buffers Work Chemistry A buffer is able to resist ph change because the two components (conjugate acid and. how a buffer works. how does a buffer work? When you add an acid to a buffer, the conjugate base. how do buffer solutions work? Buffers function through a process of chemical equilibrium. To illustrate the function of a buffer solution, consider. How Do Buffers Work Chemistry.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs How Do Buffers Work Chemistry A buffer is able to resist ph change because the two components (conjugate acid and. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. Buffers function through a process of chemical. How Do Buffers Work Chemistry.
From chem.libretexts.org
Chapter 16.6 Buffers Chemistry LibreTexts How Do Buffers Work Chemistry To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. how a buffer works. When you add an acid to a buffer, the conjugate base. buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. An. How Do Buffers Work Chemistry.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download How Do Buffers Work Chemistry An example of a common buffer is a solution of acetic acid (ch3cooh) and sodium. Ions are atoms or molecules that have lost or gained one or more electrons. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. how do buffer solutions work? A buffer is able to resist. How Do Buffers Work Chemistry.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses How Do Buffers Work Chemistry To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. Buffers function through a process of chemical equilibrium. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. how a buffer works. buffer, in chemistry, solution usually containing an acid. How Do Buffers Work Chemistry.